Deuterium Lamp vs Xenon Lamp [The Ultimate Comparison Guide]
When it comes to researching materials the ultimate choice of researchers comes down to Spectrophotometers.
As there are different spectrophotometers available in market with different specs of shapes, sizes and materials. One big factor in choosing the Spectrophotometer is the light source.
In this article we analyse most often compared two Spectrophotometer Lamp Sources Deuterium Lamps (also known as D2 Lamps) and Xenon (Xe) Lamps . The basic purpose of this article is to bring out the facts concerning these two lamps and help you make informed decision on which lamp to go for.
For comparison we have considered only pure gas based Xenon Lamps and not the Xenon Mercury Lamps.
Quick Comparison
Some of the quick facts when it comes to comparing Deuterium Lamps & Xenon Lamps are as follows,
| Factor | Deuterium Lamps | Xenon Lamps |
|---|---|---|
| Wavelength Range | 180 nm to 370 nm | 185 nm to 2000 nm |
| Spectrum(s) Covered | UV & VIS | UV, VIS & IR |
| Cost Range | Affordable | High |
Deuterium Lamp & Xenon Lamp: Characteristics in brief
A Deuterium Arc Lamp as we all know is also known as D2 Lamp is an ideal source of discharge lamp in spectroscopy. In bulbs usually the filament is the source of light but in case of D2 Lamp the arc formed is the source of continuous light. D2 lamps usually comes in handy when a continuous spectrum is needed in the ultraviolet region. The filament is usually made up of Tungsten & a continuous voltage from 300 Volts to 500 Volts is needed to fire up the filament; once the arc is formed voltage levels can be brought down.
A Xenon mimics sunlight particularly because of the range of spectrum it covers (UV, VIS & IR) and also because of the white light it emits. A typical Xenon lamp consists of anode & cathode electrodes facing each other. On passing electricity in this ionized Xenon gas this lamp then discharges light.
Detailed Comparison
Wavelength
Deuterium Lamp
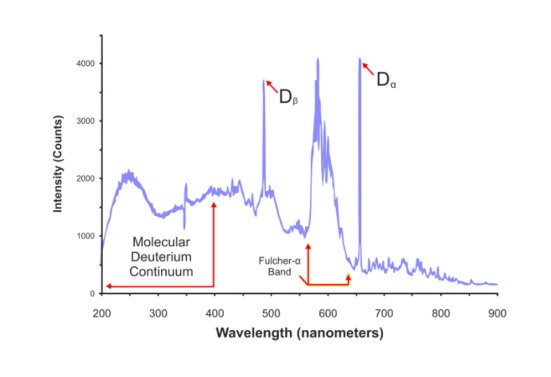
The stable and continuous is within 165 nm to 400 nm as shown in the diagram. Fulscher band emission is around 560 to 640 nm. Balmer lines are are also marked as Dβ and Dα. The balmer lines are 486 nm & 656 nm. As we move more towards the right we see more of the irregular spectra. Hence the old saying Deuterium Lamps are useful when you need stability in the UV region.
Xenon Lamp
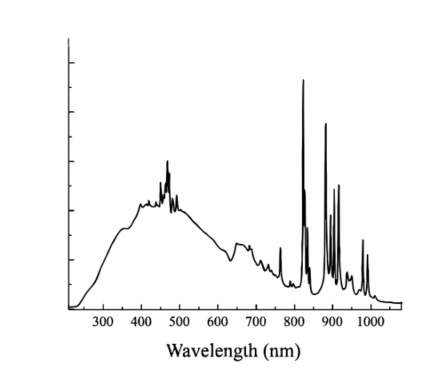
Xenon Lamps show distinctive high-intensity within spectra within 850 nm to 900 nm. The broad spectrum range shown in the figure with low stability but with long-life feature adds to Xenon lamps’ advantage.
Fluctuation
One thing that Deuterium Lamp score over other lamps is the low fluctuation levels in peak. This excellent characteristic of Deuterium Lamp at a level of 0.005% (peak to peak) although slight variation can be observed for different models. The stability in fluctuation levels is because of ceramic electrode structure found in Deuterium Lamps.
In case of Xenon Lamp the fluctuation level is of the value 1% and the value only increases in other types of Xenon Lamps.
Applications
Deuterium Lamps are common for testing purpose in Pharmaceutical industries and are also used for Atomic absorption spectroscopy (AAS), Thin film measurement and Thin layer chromatography (TLC)
Xenon lamps are commonly used as movie projectors and also for wafer inspection system and devices such as microscope.
Conclusion
Both lamps have there own set of benefits and limitations. When more of UV related stability is needed Deuterium Lamps are preferred. In situation when sunlight has to be reproduced then Xenon Lamps are used.
Xenon lamps although produces highly unstable output compared to other lamps. But with advances in lamp research better Xenon Lamps are manufactured.
Both lamps require stable power input for the arc light to be formed. In both of the lamps safety gears are advised before using them. With better brands one can get these lamps with 2000+ hours of operation.